Electrolysis Of Dilute Sulfuric Acid
The electrolysis of dilute sulfuric acid (H₂SO₄) involves passing an electric current through the solution to break it down into its elements.
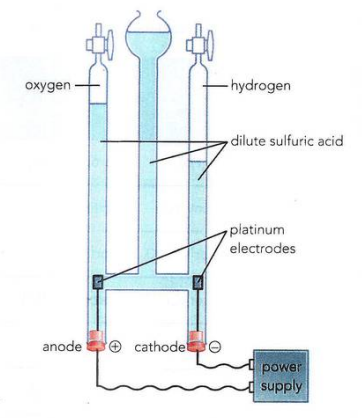
Apparatus Explanation
The liquid in the U-shaped container. It conducts electricity due to the presence of ions:

As each of the hydrogen atoms donate their electrons to the oxygen due to the tendency of 2 more electrons in its valency shell
The oxygen ions go to anode because they are negative ions and hydrogen go to cathode as they are positive ions, this is how electrolysis work within dilute sulfuric acid
Electrolysis And Electricity
Electricity is generated by electrolysis from anions, anode, cations and cathode coming together
Electricity can be caused by electromagnetic force
Electrolysis uses electricity to conduct non-spontaneous chemical reaction
Electrolysis uses the electricity and breaks the compound


















